Global Health Care Group

Immunoscore®* is an in vitro diagnostic test predicting the risk of relapse in early stage colon cancer patients, by measuring the host immune response at the tumor site.
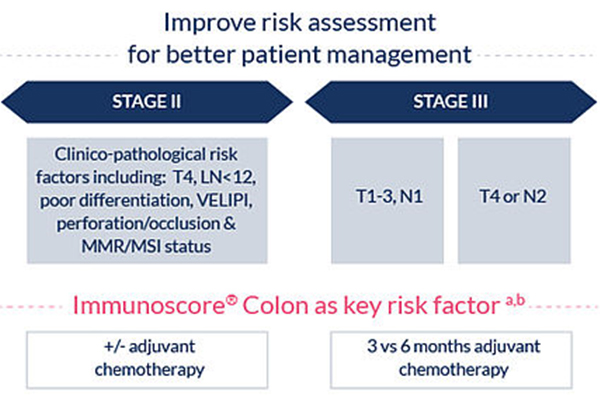
It is a risk-assessment tool that provides independent and superior prognostic value than the usual tumor risk parameters, and should be used as an adjunct to the TNM classification (Pagès F et al. The Lancet 2018, Sinicrope F et al. J Clin Oncol 2018). Immunoscore® can thus improve individual patient treatment strategies, particularly the modulation of adjuvant chemotherapy in stage II and stage III.
a) SITC International study results, The Lancet May 2018
b) N0147 study results, ASCO 17 & ASCO GI 18
* CE-IVD for European Community countries, performed in CLIA certified laboratory for the US
Material: FFPE block or FFPE slides from tumor resection
Target: CD3+ & CD8+ T cells
Location: Center and periphery of the tumor
Technology: Image Analysis
The Immunoscore® scoring has been defined in a large international SITC-led retrospective validation study conducted on more than 2500 St I-III CC patients (Pagès et al, The Lancet May 2018).
Immunoscore® under its CE-IVD version is the first immune scoring diagnostic tests used in routine by pathology labs leveraging advanced image analysis. The accuracy and robustness of the test relies on precise counting of positive immune cells in predefined regions and automatic calculation of Immunoscore® for each patient based on specific algorithm.
Immunoscore® is a test predicting the risk of relapse of patients with localized CC to help guiding treatment strategies. By evaluating the immune reaction at the tumor site, it provides independent and superior prognostic value to usual risk factors.
LARGEST CONTRIBUTION OF IMMUNOSCORE® (47%) TO SURVIVAL RISK
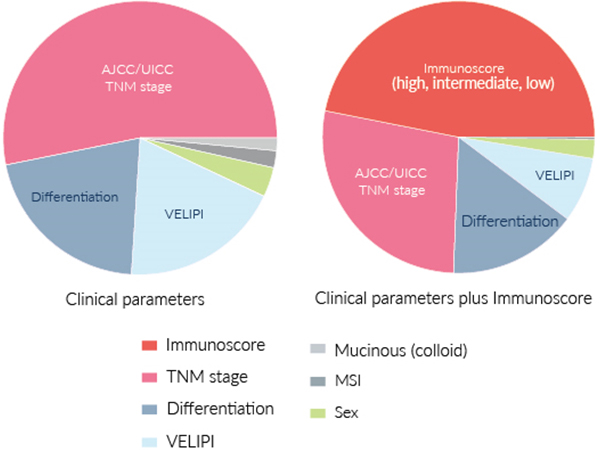
Figures extracted from Pagès F et al. The Lancet 2018
This information supports decision about adjuvant chemotherapy need (stage II) and duration of treatment (stage III).